Three species of land tortoise (Testudinidae) occur in Greece; the spur-thighed tortoise
Testudo graeca, Hermann's tortoise Testudo hermanni and the marginated tortoise
Testudo marginata. I have worked mostly on T. hermanni at a single site,
Alyki near Thessaloniki, but have also studied other populations of
all three species in northern and eastern Greece. Many of these populations were first observed
on student expeditions to Greece in 1980 and 1982. More detailed studies were
made at some of these sites by another student expedition (by Karen Barnett, Lesley Dean,
Anna Dubiel, Jeanette Lunt, Brian Moulden, Eric Steer and Jonathan Wright) in 1985, when I was
working at the University of Thessaloniki. One
particularly interesting site, Epanomi (on the opposite side of the Gulf of Thermaikos to Alyki)
was introduced to me by Vassilis Goutner of the University of Thessaloniki on a bird watching
trip. More populations have been studied by Ronald Willemsen,
from Doetinchem in the Netherlands, who has worked on Greek tortoises
since 1975. Ronald's sites are mostly in southern, central and western Greece so our studies
are largely complementary. Ronald's particular interests have been variation in
T. hermanni, and the ecology of T. marginata
which is a relatively rare species endemic to Greece (Willemsen, 1991; Bringsøe,
Buskirk & Willemsen, 2001).
 The first comparative studies dealt with habitat use and thermoregulation. It was apparent
in 1980 that there was some habitat separation between T. hermanni and T. graeca
where they were sympatric in north-eastern Greece, with T. graeca occupying more open
vegetation (Stubbs, Hailey, Tyler & Pulford, 1981). The 1985 expedition studied four sites in
detail; Alyki (the westernmost known population of T. graeca in Greece), Epanomi,
Keramoti, and Porto Lagos towards the Turkish border
(Wright, Steer & Hailey, 1988).
There was significant habitat separation at each site, with
T. graeca occupying more open vegetation as expected. Information from a range of sites
showed, however, that the habitat separation was not constant, but varied along a cline in
north-eastern Greece; T. graeca was restricted to the most open habitat (coastal heath
and dunes) at Alyki, but occupied increasingly diverse areas further east. Habitat separation
had consequences for the thermoregulation of the two species. At Alyki and Epanomi their
habitats were rather similar (coastal areas, grassland and heathland), as were body temperatures.
Body temperatures of T. graeca were similar at all four sites, where it occupied similar
open habitats. Body temperatures of T. hermanni were significantly lower in woodland
at Keramoti and Porto Lagos.
The first comparative studies dealt with habitat use and thermoregulation. It was apparent
in 1980 that there was some habitat separation between T. hermanni and T. graeca
where they were sympatric in north-eastern Greece, with T. graeca occupying more open
vegetation (Stubbs, Hailey, Tyler & Pulford, 1981). The 1985 expedition studied four sites in
detail; Alyki (the westernmost known population of T. graeca in Greece), Epanomi,
Keramoti, and Porto Lagos towards the Turkish border
(Wright, Steer & Hailey, 1988).
There was significant habitat separation at each site, with
T. graeca occupying more open vegetation as expected. Information from a range of sites
showed, however, that the habitat separation was not constant, but varied along a cline in
north-eastern Greece; T. graeca was restricted to the most open habitat (coastal heath
and dunes) at Alyki, but occupied increasingly diverse areas further east. Habitat separation
had consequences for the thermoregulation of the two species. At Alyki and Epanomi their
habitats were rather similar (coastal areas, grassland and heathland), as were body temperatures.
Body temperatures of T. graeca were similar at all four sites, where it occupied similar
open habitats. Body temperatures of T. hermanni were significantly lower in woodland
at Keramoti and Porto Lagos.
Population structure of tortoises differed substantially at these four sites, with very few
juveniles at Porto Lagos, more at Keramoti and Alyki, and many at Epanomi
(Hailey, Wright & Steer, 1988).
These patterns were shown by both T. hermanni and T. graeca, despite the different
habitat use of the two species. This is encouraging, as it suggests that observed population
structures are not substantially affected by habitat characteristics. The low numbers of
juveniles at Porto Lagos were thought to indicate a lack of recruitment and a declining
population; this area was highly disturbed. The high numbers at Epanomi indicated an increasing
population in this protected area. A recent model (Hailey & Willemsen, submitted) has built on
these observations to evaluate the ratio of juveniles/females as an index of population stability
in T. hermanni.
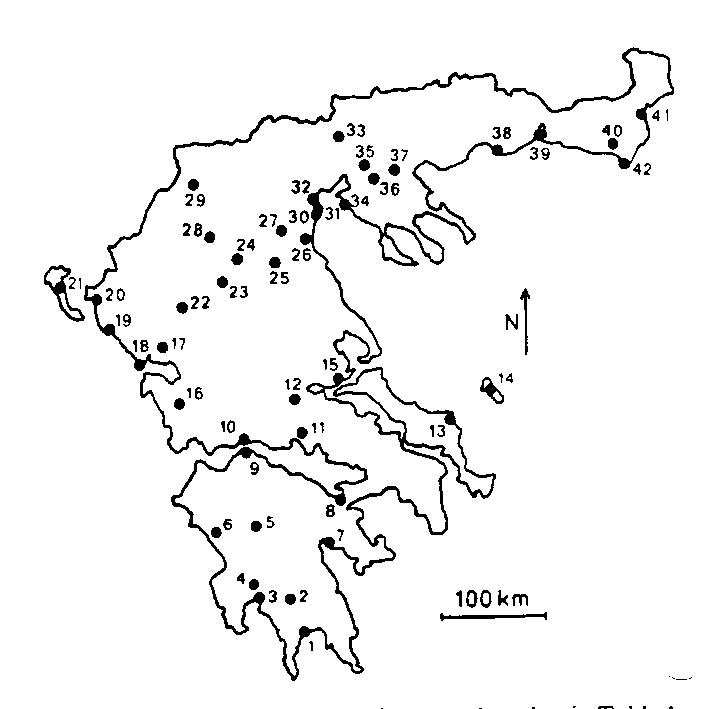 These four sites were included in a review of the status of 79 populations of tortoises at
42 sites in Greece studied by Ronald Willemsen, myself and others from 1975-1986
(Willemsen & Hailey, 1989). About a third of populations were in
immediate danger of decline, and another third faced long-term threat. There were no differences
in threat status among the three species, but populations near the coast were more threatened
than those inland. Many of these populations were visited again in spring 2001
(Hailey & Willemsen, 2003).
8/65 populations had been destroyed, a rate of extinction of about
0.8% per year. Populations that had declined or disappeared were significantly associated
with high identified threat in 1989 and with closeness to human settlement, but not with
original population density, species, area of site or characteristics of surrounding areas.
Data from 2001 also provided a test of the juvenile/female ratio as an indication of population
stability. Populations with low juvenile/female ratios were more likely to have declined in
density and to have an ageing adult population (Hailey & Willemsen, in preparation).
These four sites were included in a review of the status of 79 populations of tortoises at
42 sites in Greece studied by Ronald Willemsen, myself and others from 1975-1986
(Willemsen & Hailey, 1989). About a third of populations were in
immediate danger of decline, and another third faced long-term threat. There were no differences
in threat status among the three species, but populations near the coast were more threatened
than those inland. Many of these populations were visited again in spring 2001
(Hailey & Willemsen, 2003).
8/65 populations had been destroyed, a rate of extinction of about
0.8% per year. Populations that had declined or disappeared were significantly associated
with high identified threat in 1989 and with closeness to human settlement, but not with
original population density, species, area of site or characteristics of surrounding areas.
Data from 2001 also provided a test of the juvenile/female ratio as an indication of population
stability. Populations with low juvenile/female ratios were more likely to have declined in
density and to have an ageing adult population (Hailey & Willemsen, in preparation).
 One site discussed briefly in 1989 was Olympia, where Ronald Willemsen studied a large
population of T. hermanni from 1975-1984. Part of the site was sprayed with the
herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid
(2,4,5-T) each year from 1980. Some of the cans of herbicide used are shown to the left.
One site discussed briefly in 1989 was Olympia, where Ronald Willemsen studied a large
population of T. hermanni from 1975-1984. Part of the site was sprayed with the
herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid
(2,4,5-T) each year from 1980. Some of the cans of herbicide used are shown to the left.
 Tortoise numbers in that area apparently declined, and individuals showed symptoms of poisoning
(swollen eyes, fluid discharge from the nose and immobility).
A tortoise in the sprayed area (note blanched vegetation) is shown to the
right. Data from Olympia were recently reanalysed using the mark-recapture programs
JOLLY and SURGE to calculate numbers and survival rates
(Willemsen & Hailey, 2001).
These powerful methods confirmed that
the population in the sprayed area had declined catastrophically, with extra mortality
of adult and subadult tortoises of about 34% year-1.
Juveniles were even more strongly affected, again indicating that the juvenile/female ratio
is a good indication of a declining population. There was no difference in body mass condition
between sprayed and unsprayed areas, showing that effects were acute; mortality was not due to
starvation from loss of food plants. The scale and pattern of mortality was similar to that from
a severe scrub fire (Hailey, 2000), but spraying is potentially
more catastrophic since this is often repeated at shorter intervals than burning.
Tortoise numbers in that area apparently declined, and individuals showed symptoms of poisoning
(swollen eyes, fluid discharge from the nose and immobility).
A tortoise in the sprayed area (note blanched vegetation) is shown to the
right. Data from Olympia were recently reanalysed using the mark-recapture programs
JOLLY and SURGE to calculate numbers and survival rates
(Willemsen & Hailey, 2001).
These powerful methods confirmed that
the population in the sprayed area had declined catastrophically, with extra mortality
of adult and subadult tortoises of about 34% year-1.
Juveniles were even more strongly affected, again indicating that the juvenile/female ratio
is a good indication of a declining population. There was no difference in body mass condition
between sprayed and unsprayed areas, showing that effects were acute; mortality was not due to
starvation from loss of food plants. The scale and pattern of mortality was similar to that from
a severe scrub fire (Hailey, 2000), but spraying is potentially
more catastrophic since this is often repeated at shorter intervals than burning.
 Another phenomenon described in 1989 was the large size variation of T. hermanni in
Greece, with adult males from the north being about three times as heavy as those from the south.
This variation was recently examined more systematically in 17 of the best studied populations
(Willemsen & Hailey, 1999a).
Females were the larger sex in all populations, and the degree of sexual size dimorphism did
not vary with mean body size; females were about 2 cm longer and 1.4 times as heavy as males.
The mechanism of body size variation among populations
was differences in the duration of growth, rather than egg or hatchling size or the growth rates
of juveniles. Body size was greater in cooler areas, and increased with both latitude and
altitude. This pattern is opposite to that found in most ectotherms (the reverse Bergmann's rule),
and to that which occurs between tortoise species. The most likely evolutionary cause of size
variation between sites is differences in adult mortality, the correlation with environmental
temperature being through the frequency of fires. Greek populations of T. hermanni also
vary in colour, with those from the south having darker pigmented plastrons. A suggested
thermal advantage of this pattern has now been rejected, leaving genetic drift as the most
likely explanation (Willemsen & Hailey, 1999b).
Another phenomenon described in 1989 was the large size variation of T. hermanni in
Greece, with adult males from the north being about three times as heavy as those from the south.
This variation was recently examined more systematically in 17 of the best studied populations
(Willemsen & Hailey, 1999a).
Females were the larger sex in all populations, and the degree of sexual size dimorphism did
not vary with mean body size; females were about 2 cm longer and 1.4 times as heavy as males.
The mechanism of body size variation among populations
was differences in the duration of growth, rather than egg or hatchling size or the growth rates
of juveniles. Body size was greater in cooler areas, and increased with both latitude and
altitude. This pattern is opposite to that found in most ectotherms (the reverse Bergmann's rule),
and to that which occurs between tortoise species. The most likely evolutionary cause of size
variation between sites is differences in adult mortality, the correlation with environmental
temperature being through the frequency of fires. Greek populations of T. hermanni also
vary in colour, with those from the south having darker pigmented plastrons. A suggested
thermal advantage of this pattern has now been rejected, leaving genetic drift as the most
likely explanation (Willemsen & Hailey, 1999b).
The idea that variation of adult body size of T. hermanni in Greece is an adaptation
to differences in adult mortality rates was examined using a combination of field data on
survival and a life table model (Willemsen & Hailey, 2001).
Adult survival rates in the eight best studied populations were calculated in two ways; by
mark-recapture analysis using JOLLY and SURGE, and using growth rings. The average rate was
correlated with body size, as predicted. The life table model tested whether the variation
of survival rates was sufficient to account for the differences in adult body size, or whether
other factors (such as juvenile survival rates) were necessary. The basis of the model is the
increased reproduction of larger females (with larger clutch sizes) but the increased risk of
death before reproduction from the delayed maturity necessary to reach large adult size. There
is an optimal age at maturity and mean adult size for any given pattern of growth and survival
rates. The results of the model showed that observed growth, survival and reproduction data would
predict the observed adult female sizes.
 The life table model described above required information on the reproduction of female T.
hermanni of different body size. These data came from populations with a range of adult size,
compared to T. graeca and T. marginata, in a study with Nikos Loumbourdis of the
University of Thessaloniki (Hailey & Loumbourdis, 1988). Relative
clutch mass (clutch mass / mass of the body excluding eggs) was slightly higher in T.
hermanni (6.5-7.5%) than the other two species (4.5-5.5%). All species and populations laid
two or three clutches per year. The most interesting finding was that clutches developed in
parallel rather than serially; the energy for a clutch accumulates throughout the year and the
second clutch is already developed (in terms of energy content) when the first is laid. More
than half of the energy of the eggs has accumulated in the follicles before the female enters
hibernation. Shelled eggs have much greater volume than follicles of the same energy content.
Laying eggs in more than one clutch is probably related to the limited volume available within
the abdominal cavity; even so, food consumption may be reduced in gravid female tortoises.
Other comparative studies on Greek tortoises have examined
condition (which provided the basis of the
condition index program) and the sexual dimorphism of body size
and shape.
The life table model described above required information on the reproduction of female T.
hermanni of different body size. These data came from populations with a range of adult size,
compared to T. graeca and T. marginata, in a study with Nikos Loumbourdis of the
University of Thessaloniki (Hailey & Loumbourdis, 1988). Relative
clutch mass (clutch mass / mass of the body excluding eggs) was slightly higher in T.
hermanni (6.5-7.5%) than the other two species (4.5-5.5%). All species and populations laid
two or three clutches per year. The most interesting finding was that clutches developed in
parallel rather than serially; the energy for a clutch accumulates throughout the year and the
second clutch is already developed (in terms of energy content) when the first is laid. More
than half of the energy of the eggs has accumulated in the follicles before the female enters
hibernation. Shelled eggs have much greater volume than follicles of the same energy content.
Laying eggs in more than one clutch is probably related to the limited volume available within
the abdominal cavity; even so, food consumption may be reduced in gravid female tortoises.
Other comparative studies on Greek tortoises have examined
condition (which provided the basis of the
condition index program) and the sexual dimorphism of body size
and shape.

 Bonnet, Lagarde et al. (2001) recently discussed the shape of the
steppe tortoise Testudo horsfieldi in relation to natural and sexual selection. Interest
in sexual selection in chelonians goes back to Darwin (1871), and a neglected contemporary study
on T. graeca (Camerano, 1877). We have examined the
three European species of Testudo, and found significant sexual dimorphism in most
characters examined, the degree of dimorphism also differing significantly among the
species. Differences in shape were more likely to be the result of sexual selection than of
natural selection for fecundity, and were related to the courtship behaviour of the three
species. Prolonged mounting is probably responsible for the triangular shape of male T.
hermanni (left), and courtship of T. marginata is characterised by head movements
and severe biting (right) (Willemsen & Hailey, 2003).
Bonnet, Lagarde et al. (2001) recently discussed the shape of the
steppe tortoise Testudo horsfieldi in relation to natural and sexual selection. Interest
in sexual selection in chelonians goes back to Darwin (1871), and a neglected contemporary study
on T. graeca (Camerano, 1877). We have examined the
three European species of Testudo, and found significant sexual dimorphism in most
characters examined, the degree of dimorphism also differing significantly among the
species. Differences in shape were more likely to be the result of sexual selection than of
natural selection for fecundity, and were related to the courtship behaviour of the three
species. Prolonged mounting is probably responsible for the triangular shape of male T.
hermanni (left), and courtship of T. marginata is characterised by head movements
and severe biting (right) (Willemsen & Hailey, 2003).
Tortoises in France
I have also been involved in studies of the western subspecies Testudo hermanni hermanni
(formerly T. h. robertmertensi) in France. This subspecies is also found in Spain and
Italy; Hermann's tortoises in Greece are of the eastern subspecies T. h. boettgeri
(formerly known as T. h. hermanni) which occurs throughout
the Balkans. David Stubbs studied tortoises in the hills of the Massif des Maures, southern
France, from 1981-1983 (Stubbs & Swingland, 1985), and I visited the area several times on the way
back from my water snake study site in Spain. The tortoises occupied oak-chestnut woodland with
high vegetation cover and a relatively complete canopy. Even in summer the daily activity
pattern was unimodal, compared to the bimodal pattern in Greece with a long period inactive
at midday (Hailey, Pulford & Stubbs, 1984). Most tortoises were
found by following the sounds they made moving in leaf litter, and basking was not
observed directly. There was, however, indirect evidence of basking from body and carapace
temperatures (Pulford, Hailey & Stubbs, 1984). Some tortoises
marked by David Stubbs are still to be found in the Massif des Maures, but current research
on tortoises in the area has concentrated on more open scrub habitats in the Plaine des Maures
(Longepierre & Grenot, 1998).
 Sébastien Longepierre and Claude Grenot of the Ecole Normale Supérieure,
University of Paris, and I have worked on the ecophysiology of these
tortoises. Studies with thread-trailed tortoises showed that distances moved were similar to
those in Greece, but the areas occupied
were much larger in France (Longepierre, Hailey & Grenot, 2001).
This is probably due to the utilization of greater habitat diversity in France. The need for
reserves to include different vegetation types makes the conservation of T. hermanni in
France more difficult. Conversely, the need for large reserves increases its value as an
umbrella species for conservation of biodiversity in general. Comparison of clutch and egg
sizes and relative clutch mass in France and Greece showed that variation among sites was as great as that
between the two countries. There is no general difference between the subspecies in these respects,
but there may well be adaptive local differences between the Massif and the Plaine des Maures
(Longepierre, Grenot & Hailey, submitted). Relocation of tortoises even within France should
therefore be discouraged. We have also worked on the condition of tortoises in captivity in
France, Italy and the Netherlands (Willemsen, Hailey, Longepierre
& Grenot, 2002) and
on the water and energy balance of wild tortoises using doubly-labelled water (in preparation).
Sébastien Longepierre and Claude Grenot of the Ecole Normale Supérieure,
University of Paris, and I have worked on the ecophysiology of these
tortoises. Studies with thread-trailed tortoises showed that distances moved were similar to
those in Greece, but the areas occupied
were much larger in France (Longepierre, Hailey & Grenot, 2001).
This is probably due to the utilization of greater habitat diversity in France. The need for
reserves to include different vegetation types makes the conservation of T. hermanni in
France more difficult. Conversely, the need for large reserves increases its value as an
umbrella species for conservation of biodiversity in general. Comparison of clutch and egg
sizes and relative clutch mass in France and Greece showed that variation among sites was as great as that
between the two countries. There is no general difference between the subspecies in these respects,
but there may well be adaptive local differences between the Massif and the Plaine des Maures
(Longepierre, Grenot & Hailey, submitted). Relocation of tortoises even within France should
therefore be discouraged. We have also worked on the condition of tortoises in captivity in
France, Italy and the Netherlands (Willemsen, Hailey, Longepierre
& Grenot, 2002) and
on the water and energy balance of wild tortoises using doubly-labelled water (in preparation).
Additional references
Bonnet, X., Lagarde, F. et al. (2001). Sexual dimporphism in steppe tortoises
(Testudo horsfieldi): influence of the environment and sexual selection on body shape and
mobility. Biol. J. Linn. Soc. 72, 357-372.
Bringsøe, H., Buskirk, J. R. & Willemsen, R. E. (2001). Testudo marginata
Schoepff, 1792 - Breitrandschildkrote. In Handbuch der Reptilien und Amphibien Europas,
Vol. 3/IIIA: Schildkroten (Testudines) I (Bataguridae, Testudinidae, Emydidae), pp291-334.
Bohme, W. (Ed.). Wiebelsheim: AULA-Verlag.
Camerano, L. (1877). Dei caratteri sessuali secondari della Testudo ibera Pallas.
Atti Rea Accad. Sci. Torino 13, 97-102. translation
Darwin, C. (1871). The descent of man and selection in relation to sex. London:
John Murray.
Longepierre, S. & Grenot, C. (1998). Compte rendu de l'étude écophysiologique
de 10 secteurs dans la Plaine des Maures. Distribution de la tortue d'Hermann (Testudo
hermanni hermanni). SIVOM du Centre Var, Le Luc en Provence.
Stubbs, D. & Swingland, I. R. (1985). The ecology of a Mediterranean tortoise (Testudo
hermanni): a declining population. Can. J. Zool. 63, 169-180.
Willemsen, R. E. (1991). Differences in thermoregulation between Testudo hermanni and
Testudo marginata and their ecological significance. Herpetol. J. 1, 559-567.
 The first comparative studies dealt with habitat use and thermoregulation. It was apparent
in 1980 that there was some habitat separation between T. hermanni and T. graeca
where they were sympatric in north-eastern Greece, with T. graeca occupying more open
vegetation (Stubbs, Hailey, Tyler & Pulford, 1981). The 1985 expedition studied four sites in
detail; Alyki (the westernmost known population of T. graeca in Greece), Epanomi,
Keramoti, and Porto Lagos towards the Turkish border
(Wright, Steer & Hailey, 1988).
There was significant habitat separation at each site, with
T. graeca occupying more open vegetation as expected. Information from a range of sites
showed, however, that the habitat separation was not constant, but varied along a cline in
north-eastern Greece; T. graeca was restricted to the most open habitat (coastal heath
and dunes) at Alyki, but occupied increasingly diverse areas further east. Habitat separation
had consequences for the thermoregulation of the two species. At Alyki and Epanomi their
habitats were rather similar (coastal areas, grassland and heathland), as were body temperatures.
Body temperatures of T. graeca were similar at all four sites, where it occupied similar
open habitats. Body temperatures of T. hermanni were significantly lower in woodland
at Keramoti and Porto Lagos.
The first comparative studies dealt with habitat use and thermoregulation. It was apparent
in 1980 that there was some habitat separation between T. hermanni and T. graeca
where they were sympatric in north-eastern Greece, with T. graeca occupying more open
vegetation (Stubbs, Hailey, Tyler & Pulford, 1981). The 1985 expedition studied four sites in
detail; Alyki (the westernmost known population of T. graeca in Greece), Epanomi,
Keramoti, and Porto Lagos towards the Turkish border
(Wright, Steer & Hailey, 1988).
There was significant habitat separation at each site, with
T. graeca occupying more open vegetation as expected. Information from a range of sites
showed, however, that the habitat separation was not constant, but varied along a cline in
north-eastern Greece; T. graeca was restricted to the most open habitat (coastal heath
and dunes) at Alyki, but occupied increasingly diverse areas further east. Habitat separation
had consequences for the thermoregulation of the two species. At Alyki and Epanomi their
habitats were rather similar (coastal areas, grassland and heathland), as were body temperatures.
Body temperatures of T. graeca were similar at all four sites, where it occupied similar
open habitats. Body temperatures of T. hermanni were significantly lower in woodland
at Keramoti and Porto Lagos.
 These four sites were included in a review of the status of 79 populations of tortoises at
42 sites in Greece studied by Ronald Willemsen, myself and others from 1975-1986
(
These four sites were included in a review of the status of 79 populations of tortoises at
42 sites in Greece studied by Ronald Willemsen, myself and others from 1975-1986
( One site discussed briefly in 1989 was Olympia, where Ronald Willemsen studied a large
population of T. hermanni from 1975-1984. Part of the site was sprayed with the
herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid
(2,4,5-T) each year from 1980. Some of the cans of herbicide used are shown to the left.
One site discussed briefly in 1989 was Olympia, where Ronald Willemsen studied a large
population of T. hermanni from 1975-1984. Part of the site was sprayed with the
herbicides 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid
(2,4,5-T) each year from 1980. Some of the cans of herbicide used are shown to the left.
 Tortoise numbers in that area apparently declined, and individuals showed symptoms of poisoning
(swollen eyes, fluid discharge from the nose and immobility).
A tortoise in the sprayed area (note blanched vegetation) is shown to the
right. Data from Olympia were recently reanalysed using the mark-recapture programs
JOLLY and SURGE to calculate numbers and survival rates
(
Tortoise numbers in that area apparently declined, and individuals showed symptoms of poisoning
(swollen eyes, fluid discharge from the nose and immobility).
A tortoise in the sprayed area (note blanched vegetation) is shown to the
right. Data from Olympia were recently reanalysed using the mark-recapture programs
JOLLY and SURGE to calculate numbers and survival rates
( Another phenomenon described in 1989 was the large size variation of T. hermanni in
Greece, with adult males from the north being about three times as heavy as those from the south.
This variation was recently examined more systematically in 17 of the best studied populations
(
Another phenomenon described in 1989 was the large size variation of T. hermanni in
Greece, with adult males from the north being about three times as heavy as those from the south.
This variation was recently examined more systematically in 17 of the best studied populations
( The life table model described above required information on the reproduction of female T.
hermanni of different body size. These data came from populations with a range of adult size,
compared to T. graeca and T. marginata, in a study with Nikos Loumbourdis of the
University of Thessaloniki (
The life table model described above required information on the reproduction of female T.
hermanni of different body size. These data came from populations with a range of adult size,
compared to T. graeca and T. marginata, in a study with Nikos Loumbourdis of the
University of Thessaloniki (
 Bonnet, Lagarde et al. (2001) recently discussed the shape of the
steppe tortoise Testudo horsfieldi in relation to natural and sexual selection. Interest
in sexual selection in chelonians goes back to Darwin (1871), and a neglected contemporary study
on T. graeca (
Bonnet, Lagarde et al. (2001) recently discussed the shape of the
steppe tortoise Testudo horsfieldi in relation to natural and sexual selection. Interest
in sexual selection in chelonians goes back to Darwin (1871), and a neglected contemporary study
on T. graeca (