I went to the University of Zimbabwe in 1991, originally to work with
Jerry Patterson
on the ecology of mating systems in lizards. Jerry had been my predecessor
in Peter Davies' laboratory at the University of Nottingham, and his successful work there had
left a large reputation. Jerry became ill and left Zimbabwe three months after my arrival, and
I eventually took over his teaching in comparative animal physiology. Although there were
many interesting potential projects on the ecology of lizards in Zimbabwe, opportunities for
physiological research were limited; previous work of John Loveridge (Professor of Zoology)
and others had been on animals much smaller (insects) or larger (crocodiles) than lizards.
The facilities were, however, ideal for work on tortoises.
Before arriving in Zimbabwe I had corresponded with
Ian Coulson
of the Department of National Parks and Wild Life Management. Ian had worked on the ecology of
tortoises at the Sengwa Wildlife Research Institute in north-west Zimbabwe since 1982. The
population density of tortoises at Sengwa was low, and most were brought in to the SWRI office
by game scouts, to be measured, marked and released. About 350 leopard tortoises (Geochelone
pardalis) and 100 hingebacks (Kinixys spekii) had been marked in 10 years, population
data that would have been impossible to obtain from short-term work. We began joint research at
Sengwa in the 1991/92 rainy season, followed by visits in the next two years. I also worked
on captive tortoises in the University of Zimbabwe. Some of these animals had been kept in a
large outdoor enclosure for many years (indeed many of the leopard tortoises were captive bred).
Others were obtained following an advertisement in the Newsletter of the Herpetological
Association of Zimbabwe, courtesy of Steve Durrant, and from the Mukuvisi Woodland
Association from their park just outside Harare.
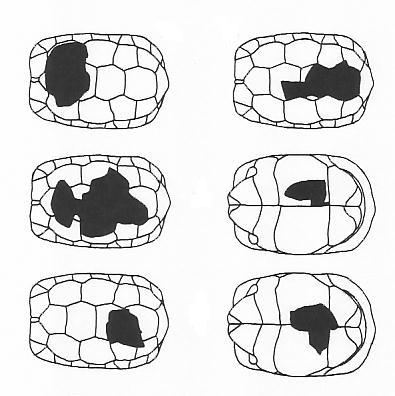
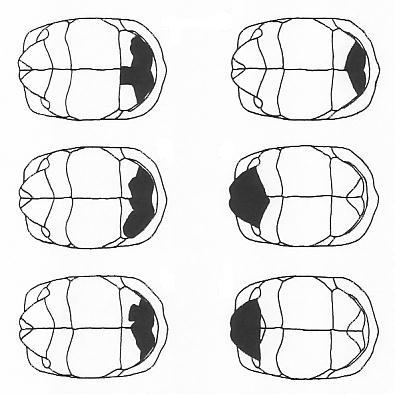 Ian's population data are chiefly of interest in being from an African environment with numerous
natural predators. The annual survival rate of K. spekii was about 0.74
(Coulson & Hailey, 2001), compared to about 0.90 for
Testudo hermanni of similar body size in Greece (Willemsen & Hailey, 2001). The mortality
rate was thus at least twice as high in Africa as in the Mediterranean, where most natural
predators are now extinct. The survival rate of G. pardalis was also low
(Hailey & Coulson, 1999a); a further paper in preparation will
describe their population ecology. Tortoises at Sengwa suffered from characteristic types of
injury, which could be attributed to large mammals (damage to the carapace margins, often
including bite marks, left) or ground hornbills (narrow holes punched in the carapace, right).
Graphical Ford-Walford analysis showed that
G. pardalis showed a non-Bertalanffy (mostly logistic-by-mass) pattern of growth, also
found in giant tortoises (Hailey & Lambert, 2002) and
other herbivorous chelonians. Bertalanffy-type
growth curves are characterised by high rates of juvenile growth relative to larger
individuals. A review of growth in chelonians suggests that Bertalanffy-type growth is found in
omnivores, which often show a decrease in diet quality with size (with the highest proportion
of animal food in juveniles), while non-Bertalanffy growth is found in herbivorous and
carnivorous chelonians in which diet quality varies less with size (Hailey & Coulson, 1999a).
Ian's population data are chiefly of interest in being from an African environment with numerous
natural predators. The annual survival rate of K. spekii was about 0.74
(Coulson & Hailey, 2001), compared to about 0.90 for
Testudo hermanni of similar body size in Greece (Willemsen & Hailey, 2001). The mortality
rate was thus at least twice as high in Africa as in the Mediterranean, where most natural
predators are now extinct. The survival rate of G. pardalis was also low
(Hailey & Coulson, 1999a); a further paper in preparation will
describe their population ecology. Tortoises at Sengwa suffered from characteristic types of
injury, which could be attributed to large mammals (damage to the carapace margins, often
including bite marks, left) or ground hornbills (narrow holes punched in the carapace, right).
Graphical Ford-Walford analysis showed that
G. pardalis showed a non-Bertalanffy (mostly logistic-by-mass) pattern of growth, also
found in giant tortoises (Hailey & Lambert, 2002) and
other herbivorous chelonians. Bertalanffy-type
growth curves are characterised by high rates of juvenile growth relative to larger
individuals. A review of growth in chelonians suggests that Bertalanffy-type growth is found in
omnivores, which often show a decrease in diet quality with size (with the highest proportion
of animal food in juveniles), while non-Bertalanffy growth is found in herbivorous and
carnivorous chelonians in which diet quality varies less with size (Hailey & Coulson, 1999a).
The ecophysiology of Zimbabwean tortoises was of special interest to me in two respects.
First, K. spekii (and other species of Kinixys) is unusual in
having an omnivorous diet, while most tortoises are more or less strictly herbivorous. Less than
half of the food of K. spekii is vascular plants, with fungi and invertebrates being
important in the diet (Hailey, Coulson & Chidavaenzi, 1997). K. spekii has a shorter gut
than the herbivorous G. pardalis, and is less able to process coarse vegetation
(Hailey, 1997).
 Fungi provide the best diet for K. spekii in terms of energy intake per unit
processing time, but are poor in micronutrients, hence the mixed diet in the field and
in laboratory trials
(Hailey, Chidavaenzi & Loveridge, 1998). Invertebrates eaten
by K. spekii are mostly millipedes (67%) and beetles. The mean prey mass was about 0.2%
of body mass, which appears small but is in fact similar to that of many insectivorous
lizards. Predatory behaviour of K. spekii is unspecialised, with tortoises killing prey
by eating them in pieces, in a similar way to plants or fungi
(Hailey, Coulson & Mwabvu, 2001).
The major adaptations of K. spekii as a predator are of the digestive system
(including the hooked beak to cut exoskeletons) rather than behaviour.
Fungi provide the best diet for K. spekii in terms of energy intake per unit
processing time, but are poor in micronutrients, hence the mixed diet in the field and
in laboratory trials
(Hailey, Chidavaenzi & Loveridge, 1998). Invertebrates eaten
by K. spekii are mostly millipedes (67%) and beetles. The mean prey mass was about 0.2%
of body mass, which appears small but is in fact similar to that of many insectivorous
lizards. Predatory behaviour of K. spekii is unspecialised, with tortoises killing prey
by eating them in pieces, in a similar way to plants or fungi
(Hailey, Coulson & Mwabvu, 2001).
The major adaptations of K. spekii as a predator are of the digestive system
(including the hooked beak to cut exoskeletons) rather than behaviour.
The second area of interest on tortoise ecophysiology in Zimbabwe was the tropical environment,
with a climate unlike the cooler Mediterranean region.
K. spekii is of similar size to T. hermanni (about 1 kg) but, apparently
paradoxically for a tropical animal, is active with much lower body temperatures
(Hailey & Coulson 1996b).
Low body temperatures serve to give a wide safety margin before critical
levels are reached, and have also been found in other small tortoises
from hot climates such as T. kleinmanni in Israel and K. belliana in Madagascar.
The climate of Zimbabwe, although tropical, is also strongly seasonal. Tortoises become dormant
in the unfavourable dry season, because of lack of food rather than unsuitable temperatures.
K. spekii shows considerable metabolic depression during dormancy
(Hailey & Loveridge, 1997), an
adaptation to a long period (7-8 months each year) spent inactive at relatively high body
temperature (and thus energetically expensive).
Thread-trailing proved to be a useful field technique in Zimbabwe, as in Greece, allowing
the distance moved by a tortoise to be measured as well as the area used. The home range of
G. pardalis was much larger than that of K. spekii in relation to body size and
daily movement distance. Widespread movements of the purely herbivorous G. pardalis allow
this species to exploit small areas of mineral-rich soil
(Hailey & Coulson, 1996a), while K. spekii obtains
micronutrients from animal food. Thread-trailing also allowed individual tortoises to be
observed repeatedly, so that their time budgets
(Hailey & Coulson, 1999b) and
thermoregulation could be studied. A developing protocol for
such studies was to compare body temperatures with temperatures of physical models placed at
random in the environment. Comparison of model and body
temperatures showed that K. spekii was thermoregulating, and that this was largely by
choice of activity time (inactivity at midday on hot days), but also by microhabitat
selection (increased use of shade in hot conditions). An extension of this protocol, comparing
body and model temperatures with those of animals in a laboratory thermal gradient, was however
found to be of limited usefulness. Although a thermal gradient provides zero-cost conditions for
thermoregulation, the temperatures selected cannot be regarded as a target for animals in the
field. The evolutionary target is the body temperatures observed in the field, which are the
result of natural selection; presumably animals with any other body temperatures have been less
successful. The concept of the effectiveness of thermoregulation, as a comparison of
observed body temperatures with a target, is therefore of only limited usefulness
(Hailey & Coulson, 1996c).
Probably the most interesting finding from this study concerned the specific dynamic action (SDA),
which is the increase in metabolic rate observed after feeding. Two theories suggest that this is
either due to metabolic work during assimilation, or to up-regulation of gut function to
prepare for assimilation. Almost all comparative studies of the SDA involve feeding a single meal,
and measuring the extra oxygen consumed above a baseline of fasting metabolic rate. The
alternative "Kellner" method (usually used in studies of livestock) involves measuring the SDA
above the baseline of a maintenance ration. The Kellner method gives a higher value of SDA;
the SDA from a single meal
is reduced as nutrients from the food substitute for those stored in the tissues, which would
otherwise have been used in metabolism, so these food nutrients do not cause an increase in
metabolic rate. Comparing the SDA from single and repeated meals provides a decisive test of
whether the SDA is due to assimilation or gut up-regulation. In the former case, the SDA would be
higher after repeated feeding, as expected from the Kellner method. In the latter case, the SDA
would be lower after repeated feeding, as the gut would already be in a fully
functional state for later meals. The first case was found; the SDA increased from 21% of the energy of
a single meal of leaves, to 42% for repeated feeding on leaves
(Hailey, 1998).
Apart from Ian Coulson and John Loveridge, these studies benefitted greatly from collaboration
with Robstein Chidavaenzi (then a B.Sc. project student, subsequently Curator of Herpetology at
the National Museum of Natural History in Bulawayo) and Taro Mwabvu
(formerly Lecturer in General Zoology at the University of Zimbabwe). The time-consuming
studies of feeding and digestion were only possible with the assistance of student research
assistants Sikhalazo Dube, Rebecca Fowlds, Davis Mumbengegwi, Regina Pawaringira, and
Ceilidh Stapelkamp.

 Ian's population data are chiefly of interest in being from an African environment with numerous
natural predators. The annual survival rate of K. spekii was about 0.74
(Coulson & Hailey, 2001), compared to about 0.90 for
Testudo hermanni of similar body size in Greece (Willemsen & Hailey, 2001). The mortality
rate was thus at least twice as high in Africa as in the Mediterranean, where most natural
predators are now extinct. The survival rate of G. pardalis was also low
(Hailey & Coulson, 1999a); a further paper in preparation will
describe their population ecology. Tortoises at Sengwa suffered from characteristic types of
injury, which could be attributed to large mammals (damage to the carapace margins, often
including bite marks, left) or ground hornbills (narrow holes punched in the carapace, right).
Graphical Ford-Walford analysis showed that
G. pardalis showed a non-Bertalanffy (mostly logistic-by-mass) pattern of growth, also
found in giant tortoises (Hailey & Lambert, 2002) and
other herbivorous chelonians. Bertalanffy-type
growth curves are characterised by high rates of juvenile growth relative to larger
individuals. A review of growth in chelonians suggests that Bertalanffy-type growth is found in
omnivores, which often show a decrease in diet quality with size (with the highest proportion
of animal food in juveniles), while non-Bertalanffy growth is found in herbivorous and
carnivorous chelonians in which diet quality varies less with size (Hailey & Coulson, 1999a).
Ian's population data are chiefly of interest in being from an African environment with numerous
natural predators. The annual survival rate of K. spekii was about 0.74
(Coulson & Hailey, 2001), compared to about 0.90 for
Testudo hermanni of similar body size in Greece (Willemsen & Hailey, 2001). The mortality
rate was thus at least twice as high in Africa as in the Mediterranean, where most natural
predators are now extinct. The survival rate of G. pardalis was also low
(Hailey & Coulson, 1999a); a further paper in preparation will
describe their population ecology. Tortoises at Sengwa suffered from characteristic types of
injury, which could be attributed to large mammals (damage to the carapace margins, often
including bite marks, left) or ground hornbills (narrow holes punched in the carapace, right).
Graphical Ford-Walford analysis showed that
G. pardalis showed a non-Bertalanffy (mostly logistic-by-mass) pattern of growth, also
found in giant tortoises (Hailey & Lambert, 2002) and
other herbivorous chelonians. Bertalanffy-type
growth curves are characterised by high rates of juvenile growth relative to larger
individuals. A review of growth in chelonians suggests that Bertalanffy-type growth is found in
omnivores, which often show a decrease in diet quality with size (with the highest proportion
of animal food in juveniles), while non-Bertalanffy growth is found in herbivorous and
carnivorous chelonians in which diet quality varies less with size (Hailey & Coulson, 1999a).
 Fungi provide the best diet for K. spekii in terms of energy intake per unit
processing time, but are poor in micronutrients, hence the mixed diet in the field and
in laboratory trials
(
Fungi provide the best diet for K. spekii in terms of energy intake per unit
processing time, but are poor in micronutrients, hence the mixed diet in the field and
in laboratory trials
(